Question
During electrolysis the reaction at anode
is?Solution
Electrolysis is the chemical process of using an electrical current to stimulate nonspontaneous reactions. A non-spontaneous reaction is one that needs energy to work while it proceeds. In other words, the process would not happen on its own, as it goes in an unfavorable, or a reversed, direction. This process requires an anode, a positively charged electrode, and a cathode, a negatively charged electrode. Oxidation occurs at anode and reduction occurs at the cathode. Hence, option A is correct.
Which of the following represents the same relation as Guitar, Flute and Music Instruments have?
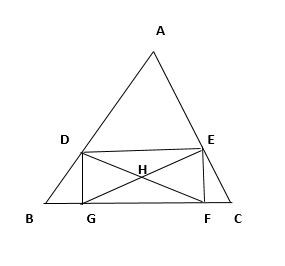
Find the number of triangles in the following figure:
Identify the diagram that best represents the relationship between mothers, women post-graduates.
Select the Venn diagram that best represents the relationship between the following classes.
Star, Sun, Mercury
Chairman , Brother , Human Being
How many people belong to W group but not X group?
Identify the correct mirror image of the following figure when mirror is placed to the right of the given figure.
Which of the answer figures illustrates the relationship between: Delhi, Sri Lanka, Asia?
‘BRIGHT’ is related to ‘ZEFGPR’ in the same way as ‘FINGER’ is related to ‘___’.
Relevant for Exams:


